How does carbon capture work?
Carbon capture technology, first developed by oil and gas companies, may be essential to fight climate change. How do these technologies work?

Carbon capture is just what it sounds like: the process of trapping carbon emissions before they enter the atmosphere.
There is a suite of technologies that can accomplish this, and many have been used for decades—even before we fully understood the climate crisis. That's because they were not pioneered not as climate-change solutions. Quite the opposite, in fact.
A recent study found that the public is, generally, not very well informed about carbon removal. So let's break down this complex technology and understand how a tool of the oil-and-gas industries have become an unavoidable—though troubled—solution to climate change.
📚 Jump to section:

How is carbon captured?
At its core, carbon capture is simple: CO₂ is piped away from an industrial facility then on to storage. The hard part is separating the CO₂ from other waste gases.
This can be accomplished by passing industrial waste gases through or over various chemically treated surfaces: liquid solvents or solid materials dotted with chemicals known as "sorbents" or specially designed membranes. In each case, chemicals bind with the CO₂, holding it in place while other gases escape.
Most industrial carbon emissions come from the combustion of fossil fuels. Combustion is a chemical process: the fuel is heated in the presence of oxygen until molecular bonds break, releasing energy. This process has a few waste products, including CO₂. The simplest way to separate the CO₂ is, then, after combustion. This is known as "post-combustion capture."
In some facilities, the fuel is actually treated before combustion, undergoing a few different chemical changes that do not release its latent heat. Essentially, the fuel is converted into a combination of hydrogen (H₂) and carbon dioxide (CO₂). The CO₂ can be separated, and the H₂ becomes a new source of fuel. H₂ is considered a "clean" fuel, since the only waste product is water vapor, H₂O. This process is known as "pre-combustion" capture, and tends to be more efficient, but also more expensive to implement, than post-combustion capture.
There is a third common process, known as "oxy-fuel combustion." Typically, fuels are combusted in ambient air, which contains high levels of nitrogen. In oxy-fuel combustion, the fuel is burned in pure O2. This simplifies the waste stream, increasing the concentration of CO₂ and thereby making it easier to capture.

Once carbon is separated, the challenge isn't over. The carbon has to be put somewhere so that it won't escape back into the atmosphere. (That's why this technology is generally known as "carbon dioxide capture and storage" or, sometimes, "carbon dioxide capture and sequestration," both of which can be shortened to CCS.) Generally, CO₂ is compressed and transported by pipeline to a disposal site. Today, disposal sites are underground, where surrounding rocks help hold the gas underground.
Where does the technology stand?
These processes have been used for decades, and were developed by oil-and-gas companies as ways to turn bigger profits. CO₂ separation is used as a way to refine natural gas so that it can be shipped and stored. The first attempts to "store" CO₂ underground, meanwhile, were aimed at extracting oil: the CO₂ goes into underground wells so the oil will be pushed out. This process, known as "enhanced oil recovery" or EOR, has been used for nearly 40 years. (And, though EOR has detrimental environmental impacts, it is different than fracking, which actually breaks apart underground geology in pursuit of the oil.)
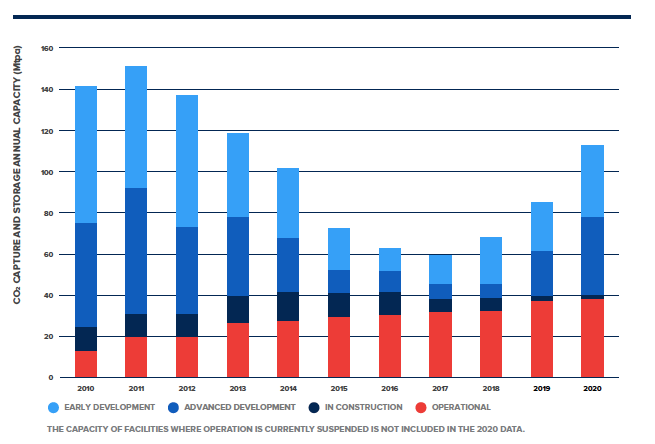
The Global CCS Institute, a think tank that supports the technology's development, reports 26 commercial CCS projects in operation as of 2020. Together, these projects have the capacity to capture and store nearly 40 million tons of CO₂. But nearly half of these are focused on refining natural gas. There are only two examples of major carbon-capture projects attached to electrical plants: the Petra Nova coal plant in the U.S. and the Boundary Dam coal plant in Canada. Both capture CO₂ after combustion. (Seven more power plants are in what the Global CCS Institute considers "advanced development," with two scheduled to come online before 2025.)

What about carbon removal?
At this point, we need to do more than stop carbon from reaching the atmosphere—to hold warming below 1.5°C, some carbon will have to be removed from the atmosphere. Carbon removal builds on carbon-capture technology, but instead of waste streams, it's the air in the atmosphere that is processed.
At this point, we need to do more than stop carbon from reaching the atmosphere. Some will have to be removed.
The high-tech route is known as "direct-air capture" (DAC), and the technology exists already. Climeworks, a Swiss company, recently became "the first direct-air-capture venture in history seeking to sell CO₂ by the ton," according to the New York Times. Climeworks uses giant fans to pull air into ducts, where a specially engineered surface attracts and binds to the CO₂ molecules. (Climeworks is too small to be considered a major project by the Global CCS Institute.) A few other DAC projects are under development, as well.

DAC is currently very energy intensive, which makes it quite expensive. If powered by fossil fuels, DAC can only be counterproductive, and some researchers note that there is no viable market for the captured carbon. (Right now, the value of captured CO2 is mostly just in its use for extracting more oil, which explains why the world's largest DAC facility is being built by a fossil-fuel company.)
There is a lower-tech method for removing carbon dioxide, too: simply spreading the dust of certain rocks, like basalt, atop farm fields. Known as enhanced rock weathering, this process relies on the rocks' ability to bond with carbon as it degrades. A recent study suggests this could be our best near-term method of carbon removal. (For a detailed look at various carbon removal strategies, see this excellent primer.)
Combining technology and nature
Then there is an even more familiar low-tech removal method: trees (and other plants), which through photosynthesis consume carbon dioxide. Much of the carbon plants pull from the air is stored inside their cells. Combine this with carbon-capture technology and you have a potentially carbon negative form of energy.
Here's how it works, in short: trees or crops are planted, and throughout their lives remove carbon from the atmosphere. Then, once harvested, these plants are converted into biofuels, such as liquid ethanol or wood pellets. These biofuels are burned in facilities outfitted with carbon-capture technology, so that the carbon in their cells does not return to the atmosphere.

This combination is known as "bioenergy with carbon capture and storage," or BECCS, and is currently much cheaper than DAC. Though there are still questions about its implementation, particularly around land use: anywhere we grow plants for fuel is space where people can't live or grow food. Some projections suggest we'd need as much land as all of Australia to pull off BECCS at the needed scale. Sometimes, too, harvesting plants for fuel leads to destroying ecosystems that would better store carbon if left alone. Drax, a United Kingdom power company has begun to convert a coal plant into a BECCS plant that burns wood pellets—but it has been criticized for sourcing wood from vulnerable forests in the Southern United States.
Further breakthroughs in carbon storage
If we abandon fossil fuels—as we need to—then CO₂ for enhanced oil recovery will no longer make sense. So where will the carbon we remove go? Already, some companies are injecting carbon into pockets of saline water in of underground rocks. Some of the CO₂ dissolves into the water, and then reacts with the rocks, helping to ensure the CO₂ stays trapped underground.
Depleted oil and gas reservoirs may be another important storage site, though their extent and effectiveness are still being studied. Another possibility involves allowing the gas to react with the rocks directly. Certain minerals are known to interact with CO₂ so that the carbon quickly converts to an immobile mineral.
Soil, rather than rocks, may also be an effective storage site. Biochar, which is essentially just charcoal, can be spread atop soil to help increase its carbon content—while also improving the soil's health.
The ocean is also a natural carbon sink, but it has generally been disregarded for storage, since CO₂ makes the water more acidic, causing damage to ecosystems. Still, scientists are investigating potential methods of storing CO₂ in very deep ocean water or in the sediments on the ocean floor.
There is also emerging interest not just in carbon storage but carbon "utilization." Carbon dioxide can be sent into greenhouses or used to carbonate sodas. Or it can be used as an input for a new process, producing anything from concrete to limestone to baking soda.
A thorny but necessary solution
There are clear ethical and environmental questions raised by carbon capture and carbon removal, but these are questions we must face as we transition away from fossil fuels.
Carbon capture has always been bound up with the fossil fuel industry, and in almost every case today, it simply reduces the emissions produced by fossil fuels, rather than eliminating emissions entirely. That's why some critics call CCS a "Trojan horse" for oil-and-gas companies. They worry that its use will tempt us to continue burning fossil fuels, rather than finding renewable sources of energy. And it is clear that we need to abandon fossil fuels as quickly as possible.
Carbon capture plays its most important role not in power generation, but in reducing industrial emissions.
Where carbon capture has an important role is not in power generation, but in industry. Industrial activities contribute 25% of global emissions, according to the Intergovernmental Panel on Climate Change, and some of these are "process" emissions—produced, that is, not by burning fuel, but by the chemical reactions used to create a product. Cement is a major example. Researchers are exploring new and cleaner production processes as well as potential low-emission replacement materials for cement. For the time being, though, capture technology is the only proven method for decreasing cement emissions at a major scale. Unfortunately, no major cement production facility currently employs carbon capture.

What about carbon removal? This technology could be useful even after we transition away from fossil fuels, because it will allow us to undo some of today's emissions. And it's clear that we will have to remove something: the IPCC could not find a pathway that limits warming to 1.5°C and avoids carbon removal entirely.
Given how little we know about how the technology can scale, the IPCC believes that "reliance on such technology is a major risk in the ability to limit warming." Some critics point out that the risk largely falls on the most vulnerable. If carbon removal does not pan out, it's the global poor who will suffer the most.
This all offers an important reminder: while we must pursue this technology, we can't depend on it alone. We cannot, in other words, just keep on living how we're living and how that all our emissions will be removed. We need to reduce emissions now, while working on every technology that might help us down the road.

